
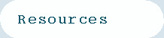





PART 1. Separation of Ions with FAIMS
FAIMS does 3 things, and each will be explained separately: What can FAIMS do?
(1) FAIMS can separate ions from each other.
(2) FAIMS can focus ions
(3) FAIMS can trap ions in 3-dimensions
The following
is a simple description of ion separation in FAIMS. A more complete description
can be found in:
"Atmospheric pressure ion focusing in a high-field asymmetric waveform
ion mobility spectrometer", Review of Scientific Instruments, 70 (1999)
page 1370.
If a mixture of ions of different sizes and types is introduced between two metal plates, the application of high voltage in an appropriate waveform to the plates will create a condition where some types of ions drift and hit the metal plates while other types of ions remain between the plates. This is the separation of ions which we can achieve with FAIMS.
The separation of the ions between the plates will require the application of a specialized waveform which we call an 'asymmetric' waveform. For example, this waveform can be a square wave in which a high positive voltage is applied for a short time and a low negative voltage is applied for a longer time. However, the area (voltage x time) of the positive and negative portion of the wave must be equal. The peak voltage of the waveform is called the 'dispersion voltage', or 'DV' for short. Diagram 1 shows an example of the waveform.
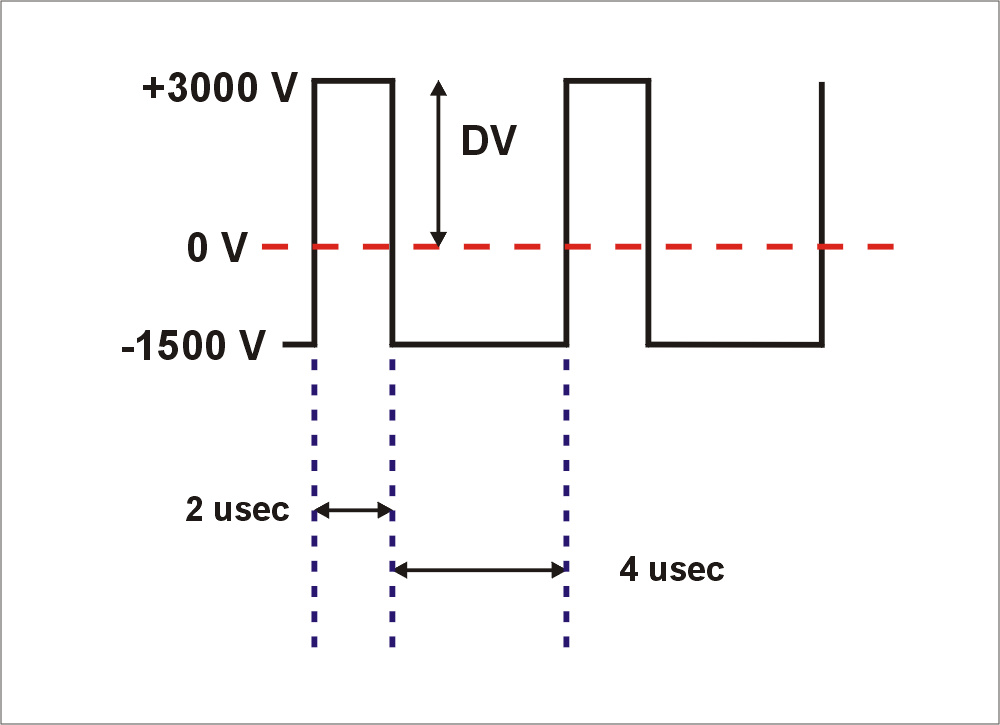
Diagram 1. Example of an asymmetric waveform.
If the electric fields which are created by the application of the asymmetric
waveform are weak, for example the field never exceeds 200 volts/cm, the ion
will move back and forth, or oscillate, during the waveform, but the ion will
not be pushed towards either plate. If the electric field during the high-voltage
part of the waveform is above about 5000 volts/cm, then the application of
the waveform may cause the ion to drift in one or the other direction towards
one of the plates. Under these conditions, during the high voltage period, the mobility of the ion may deviate from its low-field value. Diagram 2 shows examples of ions drifting towards the plates.
In the middle picture, the ion is in a 'balanced' condition which will
be described below.
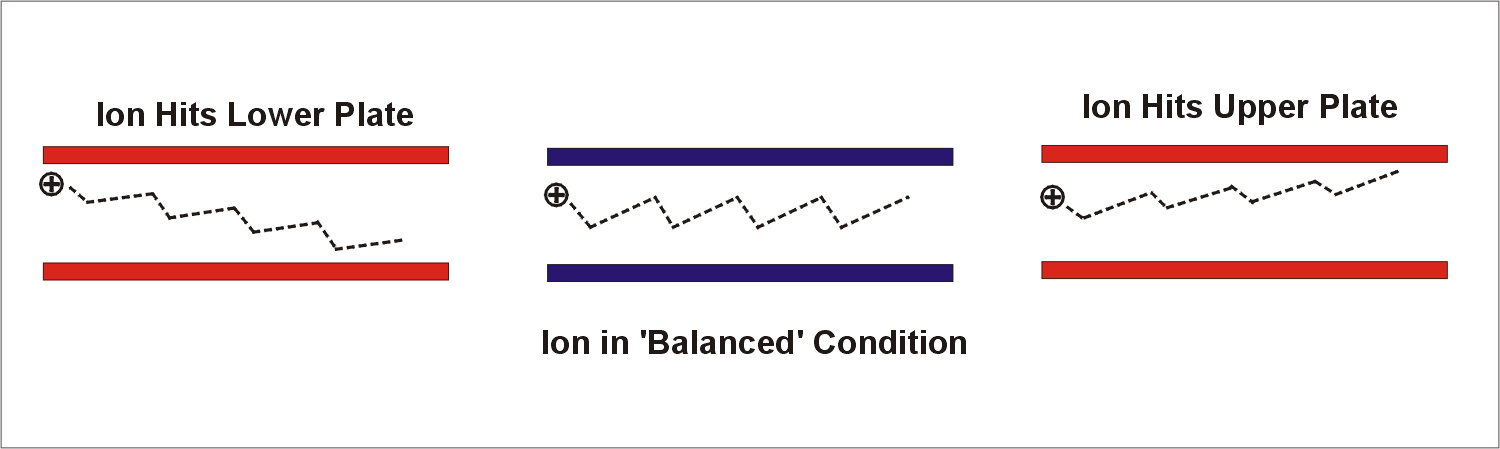
Diagram 2. Ions oscillating between the FAIMS plates.
An ion must be in the 'balanced' condition to exit from FAIMS. The reason the ion will drift towards a plate is that the ion mobility during the application of the high electric field is different than the ion mobility during the low electric field. Since the mobility of the ion defines how fast the ion moves in the field, the ion will move 'proportionately' farther in one field than the other. For example, assume that the mobility of the ion is 2 cm2/Vsec in the low field and 2.1 cm2/Vsec in the high field. The differing ion mobilities will result in a higher-than-expected velocity during the application of the high field and will result in a higher-than-expected distance traveled during the high field portion of the waveform. Since this waveform is applied at high frequency (over 200 kHz), the small extra distance traveled during each high-voltage period translates into a net drift of the ion towards one of the plates.
Some ions will have a mobility which increases with electric field; other ions have a mobility which decreases with field. These ions will travel in opposite directions between the plates.
Certain ions, such as the chloride ion in nitrogen or oxygen, experience a very large change in mobility with field. During the application of the asymmetric waveform, the chloride ion will drift very rapidly towards a plate. On the other hand, some ions, such as the tetrapropylammonium ion, experience a very small change in mobility with field and drift slowly towards one of the plates.
The drift of an ion towards one of the metal plates can be stopped by the application of a small dc voltage to either of the plates. If the voltage is applied with the appropriate magnitude and polarity, the ion will feel the force of this field and the drift of the ion will be stopped. We call the voltage which is applied to reverse or compensate for the ion drift, the 'compensation voltage' or 'CV' for short. The voltage necessary to stop the drift of the chloride ion will be high, since the mobility of this ion increases substantially at high electric field. On the other hand, the CV necessary to stop the drift of the tetrapropylammonium ion will be small. The CV is therefore the 'handle' we use to control the separation of ions.
If a mixture of ions is placed between plates, and a high voltage asymmetric waveform is applied, e.g., DV=3000 volts on plates 2 mm apart, then the different types of ions will begin to migrate towards the plates at rates that are characteristic of those ions. If a CV is applied, most ions will hit the plates except the ions for which the CV is exactly the right voltage to 'balance' the drift caused by the application of the asymmetric waveform. The mixture is therefore separated. We can select the types of ions which are in a 'balanced' condition between the plates by adjusting the voltage used for CV. This is illustrated in Diagram 3. A mixture of ions carried by a gas flow can be resolved into several peaks by scanning CV and simultaneously detecting the ions successfully transported through the gap between the plates. Each type of ion travels through the plates at a specific characteristic value of CV. The 'spectrum' of peaks observed in this manner is called a CV spectrum.
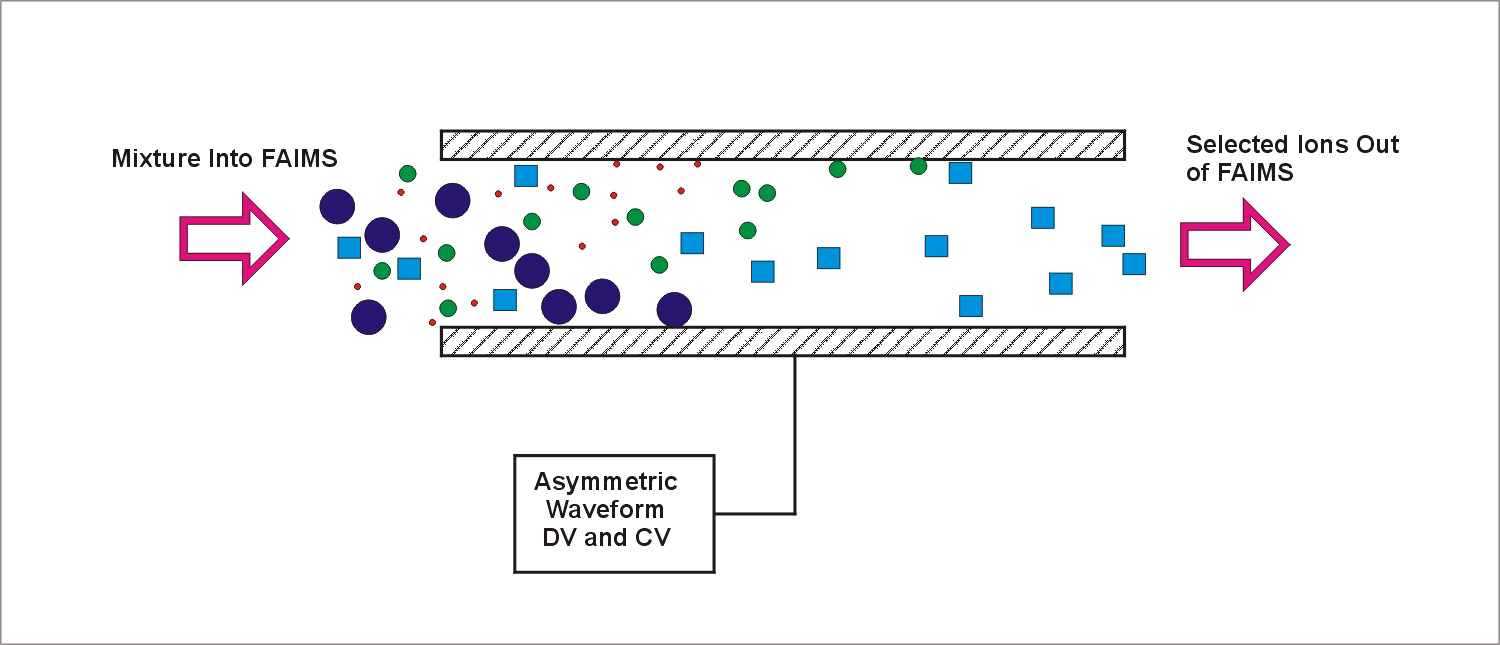
Diagram 3. Ions transmitted through FAIMS at a fixed DV and CV. Only ions in a 'balanced' condition can successfully travel through the plates.