





PART 3. Selected issues related to the application of FAIMS
This PART 3 covers some of the experimental aspects of FAIMS. In the first section we consider several factors including the waveform shape and frequency and the electrode temperature. In a second section we deal briefly with resolution.
I. Electric field strength
Figure 1 reminds us that the electric field strength is important in separation by FAIMS. The traces indicate the observed compensation voltage(CV) at various applied dispersion voltages(DV) for two charge states of a selected peptide. Firstly the CV of transmission of these ions increases with applied DV. Secondly, the CV's of the two ions become more different from each other as the DV increases.
The lower pair of traces in Figure 1 were collected with electrodes spaced apart by 2.5 mm, whereas the top traces were collected using electrode spacing reduced to 1.25 mm. Increasing field strength as before, increases the observed CV and the ion separation.
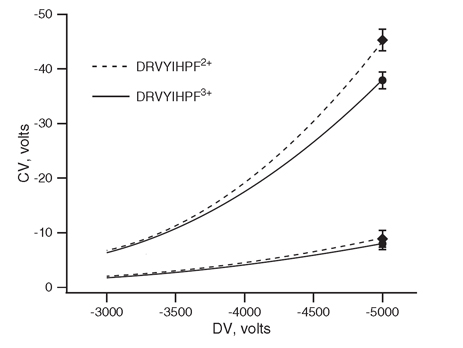
Figure 1. The effect of field strength on observed CV. Upper traces were collected with 1.25 mm electrode spacing and the lower pair with 2.5 mm spacing.
II. Waveform shape and frequency
The waveform shape in FAIMS must be asymmetric, having the peak voltage different for the positive and negative halves of the waveform. The applied waveforms are usually a square wave, or a square wave approximated by addition of two or more sinusoidal waves. The fundamental FAIMS effect is based upon the difference in mobility of the ion at a low and a high strength electric field. Clearly the effect is maximized if each field strength low or high, are held constant for the duration of their part of the waveform. Any waveform that is less perfect than a square wave, including sinusoidal approximations, will be less effective.
The waveform shape will control the relative difference in the electric field during the high and low field portions of the wave. If the waveform is composed of high and low field portions having similar duration times, the difference between the two field strengths will be small. As the wave becomes more asymmetric, and the difference in electric field strengths becomes larger, the FAIMS effect, and ion separation, will increase. However this does not continue indefinitely because as the period of high voltage becomes shorter the drift distance diminishes and the FAIMS effect again diminishes.
In addition, altering the waveform towards higher voltage is limited because an electrical discharge will eventually occur. Assume that Edis is the strongest field possible using the hardware configuration and bath gas composition selected, and thi and tlow are the periods of time at high and low field. The ratio of the time durations, thi /tlow must be equal to Elow / Edis but beyond this the user must select the value of this ratio and must establish the waveform frequency.
The effect of waveform frequency is also less clearly defined. At the low extreme of frequency, the ions travel sufficiently far during one period of the waveform that collision with the wall is inevitable. At frequencies sufficiently high that the ion travel is a small percentage of the electrode spacing it is expected that ion transmission may be adequate. At very high frequency new effects may come into play. For example time is required for the ion to reach a velocity appropriate for the applied field strength. In some cases, for example if ion solvation is a factor determining the final ion velocity, sufficient time is required for the number of collisions that permit the ion to be suitably stably solvated. At frequencies that are too high, the FAIMS effect and separation will diminish. Normally however, the production of high frequency - high voltage waveforms is limited by the electronics available to produce the waveform.
III. Electrode configuration
There are two different electrode configurations used in FAIMS devices used for mass spectrometry. The ions may be separated in regions having either (a) curved electrodes or (b) flat electrodes. Curved electrodes are beneficial because they focus the ions together to minimize losses to walls caused by diffusion and ion-ion repulsion. Because ion loss is minimized, the devices can be larger and ion separation can be at modest speed. Flat devices are subject to the full detrimental effects of diffusion, therefore the separation must be fast to avoid ion loss to walls. In order that the separation will proceed quickly, the space holding the ions must be small so that the ions which are being rejected will be rejected quickly, retaining as many of the ions of interest as possible. Fortunately the onset of electric discharge occurs at stonger fields in smaller spaces, thus permitting the required higher electric field strengths.
Fortunately, both of these approaches work well, and both have been used successfully.
IV. Bath gas composition
Figure 2 illustrates an example of the effect of changing the composition of the bath gas. As in Figure 1 we are considering ions of different charge states of a peptide. As before, we are interested in the behavior of these ions as the applied voltage, DV, is increased. The lower of the traces were recorded while pure nitrogen was used as the bath gas. The upper traces were recorded while a 1/1 mixture nitrogen and helium was used as the bath gas. In this example the addition of a new gas, helium, significantly altered the CV's of transmission of these ions as well as slightly improving separation. It is also interesting to note that the doubly charged peptide is transmitted at a higher CV than the triply charged in pure nitrogen, whereas it is transmitted at lower CV than the other in a mixture containing helium. Why?? Many questions remain unanswered.
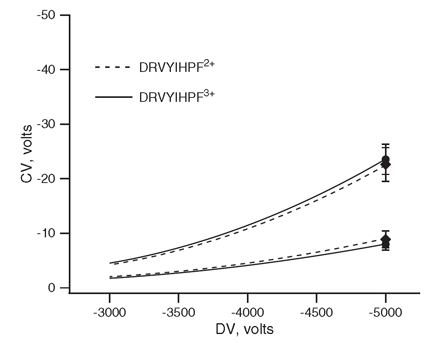
Figure 2. The effect of gas composition on observed CV. Upper traces were collected with a 1/1 mixture of N2 and He, and the lower pair using N2 [Barnett et al].
Carrier gas mixtures containing H2 improves FAIMS resolution for the same reasons as does He. However, the key advantage of H2 is an electrical breakdown threshold that greatly exceeds that for He. This extension of applied DV often results in higher CV and better separation. Clearly, however, the flammability of H2 requires stringent safety precautions be taken.
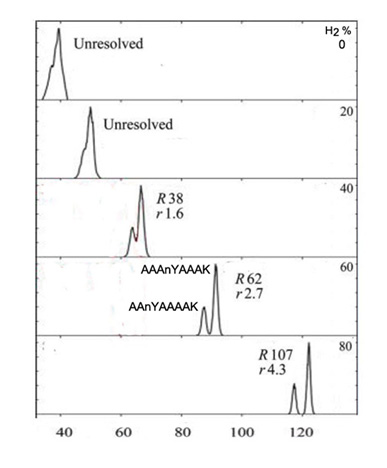
Figure 3. Separation of tryptic peptides using mixtures of N2 and hydrogen. Shown is separation of the 1+ protonated isomers AAnYAAAAK and AAAnYAAAK (with nitrotyrosine, nY, shifted by single residue)(both 782 Da) using N2 with 0-80% H2 at filtering times of 0.2 s. When separated, AAnYAAAAK appears at lower CV. Shvartsburg and Smith
Gas mixtures that include solvent vapour have been found to exhibit very interesting results Rorrer and Yost. Unusually high CV values resulted from use of a bath gas doped by solvent vapour. These high CV values suggest that complexation between the ion and solvent molecules takes place during the low-field portion of the waveform. The resulting decrease in ion mobility enhances the difference in mobilities during the low-field and high-field parts of the waveform, thus increasing CV. There were substantial benefits provided by adding solvent vapor, including dramatically increased resolving power of isomeric ions (up to ∼141). Specifically, analyte ions examined in dried nitrogen exhibited overlapping, undistinguishable CV values, but with large CV shifts afforded by addition of solvent vapor, these analyte ions were completely resolved from one another.
V. Temperature
The temperature of each of the two FAIMS electrodes plays a role in ion separation. Normally, with closely spaced flat plate FAIMS, the two plates are held at the same temperature. The selected temperature, usually slightly elevated, will ensure that the electrodes remain clean and free of surface layers. Clearly, the temperature is held low to prevent chemical modifications to the analyte in the case of biological samples.
In the case of curved electrodes the electrodes are often held at different temperatures [Barnett et al]. The temperature difference affects the ion separation. Normally the electrodes are held about 20oC difference, with the electrode with smaller radius held at the lower temperature. The magnitude of this temperature difference will modify ion separation, device resolution, and ion transmission efficiency, and will be determined experimentally for the compounds under investigation. Figure 4 illustrates the separation of two compounds at different combinations of electrode temperatures. Note that the separation is better when the inner electrode is held at the lower temperature.
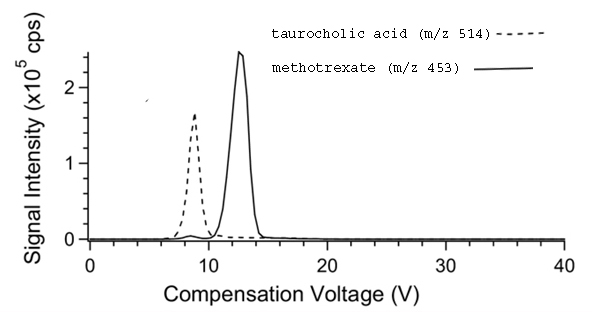
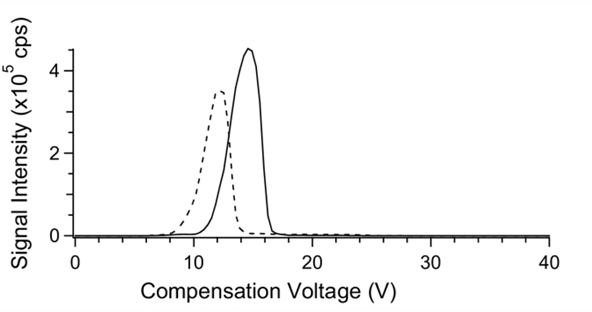
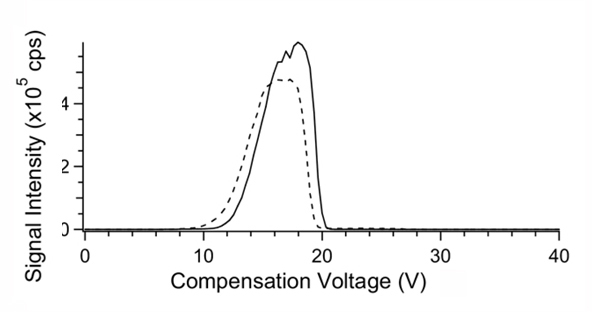
Figure 4. The effect of temperatures of the inner and outer electrodes in concentric cylinder FAIMS on the CV spectrum of the ions of taurocholic acid (m/z514) and methotrexate (m/z 453). Inner and outer electrode temperatures were: (top) 36oC/76oC, (middle) 76oC/76oC, and(bottom) 116oC/76oC.
As discussed above, the temperature is generally kept sufficiently low to prevent chemical modifications to any labile species in the sample. On the other hand, low temperatures often promotes formation of solvated ions during the low field portion of the applied waveform. In some cases formation of these complexes further increases the observed CV, and results in improved separation.
VI. Time
Time is an element that affects the ion separation in FAIMS in complex ways. At the simplest level, ions set in motion with differing electric fields would steadily become farther apart in time. Unfortunately several other factors influence the ions in addition to the electric field. Examples include diffusion and ion-ion repulsion, both of which disperse the ion cloud in time. Clearly two groups of ions set in motion to be separated, will never escape each other if the rate of diffusion keeps the two clouds of ion interspersed among each other.
The difference in mobility of ions in high and low fields will, in some cases, be a result of association on the ion with bath gas molecules or other (eg solvent or impurities) components in the carrier gas mixture during the low-field half of the waveform Leonard C. Rorrer III and Richard A. Yost. Clearly such complexation reactions require time. The degree of association of the ion with other molecules depends on: (a) concentrations of the appropriate species in the gas, (b) the complexity and kinetics of the complexation, and (c) time. At low waveform frequency the ion will have more time during the low-field part of the waveform for the complexation reaction to proceed. Furthermore, the electric fields that the ions experence will dictate the degree of complexation. If the complex is able to form during the low-field half of the waveform, and completely dissociates during the high-field portion of the waveform the difference in mobility in the two parts of the waveform will be maximized, and CV wll be larger.
Time also plays a role in determining the fraction of the original ions that will ultimately be detected. A good separation process that takes so long that all ions are lost by collision to the walls is useless. Ions are lost from small spaces rapidly. This means that if the ions are confined in small spaces the separation must be completed quickly. This also requires that the ions must be subjected to strong forces so that they rapidly separate from each other.
VII. Other effects
Ion separation in FAIMS is subject to other effects including ion-ion repulsion (space charge effects), ion diffusion, and bath gas turbulence, to mention only a few. Unfortunately these effects (in conjunction with time) never assist in the separation, rather they always try to thwart and undermine the separating process.
As discussed above in 'time' each of the two approaches, curved and flat electrodes, of FAIMS tries to minimize some of these negative components, but a final solution will gradually appear as each is further developed.
Resolution of FAIMS devices
Many of the same parameters discussed above in relation to separation, are also relevant to resolution.
In general, in a FAIMS with curved electrode geometry, the peaks get wider as the CV and DV are increased. By comparison, in a FAIMS of planar geometry the peak width remains constant or decreases while the applied voltages CV and DV are increased. In principle therefore the resolution advantage of planar geometry over curved electrodes increases as the CV and DV of ion transmission increase.
Why do peaks in a FAIMS with curved electrode geometry get wider as the CV and DV increases? The resolution of FAIMS constructed from curved electrodes is limited because the field strength between the electrodes is not constant. This range of field strength permits a type of ion to be transmitted successfully over a range of applied CV values. The CV peaks are therefore wide. On the other hand the fields between parallel plates is constant, significantly decreasing the range of CV's suitable for transmission of each type of ion. The parallel plate FAIMS has higher resolution than a curved plate version.
The search for increased resolution is unending. How can resolution be enhanced? The first option is to increase the applied DV, which results in more widely separated CV values. However the electrical breakdown of the gas prohibits unlimited increases of voltages. This breakdown can be moved to higher fields by a decrease of the gap between electrodes (Paschen law). Clearly, this narrow space always corresponds to decrease of ion transmission via losses through diffusion. Narrow gaps are routinely used with parallel plate FAIMS, The ion transmission losses are reduced by fabricating a FAIMS consisting of an array of numerous closely spaced electrodes Owlstone.
The second approach to increase resolution is to modify the composition of the carrier gas. Clearly a mixture of nitrogen and helium has the effect of increasing the observed CV of ions without the increase of the asymmetric waveform voltage (DV). This increase of CV results in improved resolution. A gas mixture of nitrogen and hydrogen Shvartsburg and Smith has an analagous and further enhanced extension of observed CV of ions. Though hydrogen may be more widely available and less expensive than helium, the dangers of utilization of this gas are well known.
This second approach has been extended. It has been found that small percentage additions of solvent vapours Rorrer and Yost to the FAIMS carrier gas results in remarkably significant increases in observed CV vales, The CV of some ions can be moved over 50V higher by small addition of vapours. This approach has the potential of significantly increasing FAIMS resolution. It has been postulated that the solvent vapour permits the ions to be complexed during the low-field portion of the waveform, and this cluster dissociated to yield free ions during the high-field part of the waveform. The difference in mobility of the free ion and the complexed form of the ion can be significant. This direction offers interesting research possibilities.
A third approach to improve resolution of parallel plate FAIMS involves extension of the ion separation time Shvartsburg and Smith. The FAIMS electrodes may be made longer, permitting more time for separation, while maintaining a constant gas flow. This option suffers the disadvantage of loss of ion transmission through collisions with electrode walls. However the increase of ion separation capability, especially applied to complex sample mixtures, may outweigh the loss of sensitivity.