





PART 4. Examples the application of FAIMS
Some selected examples are considered here. We will concentrate our attention on those things that FAIMS can do that other technologies cannot.
Separation of enantiomers.
These are of special significance since the mass spectrometer might have difficulty with these compounds. Compounds whose isomers are non-superposable mirror images of each other, referred to as chiral compounds, have identical masses and cannot be separated by mass spectrometry. It has been shown that amino acid enantiomers can be separated by complexation and electrospray ionization (ESI) followed by FAIMS, In this example the drug terbutaline is complexed in a copper containing triad (Cu(L-Trp)2(terb)-H) and separation is shown in Figure 1. (Axel Mie et al)
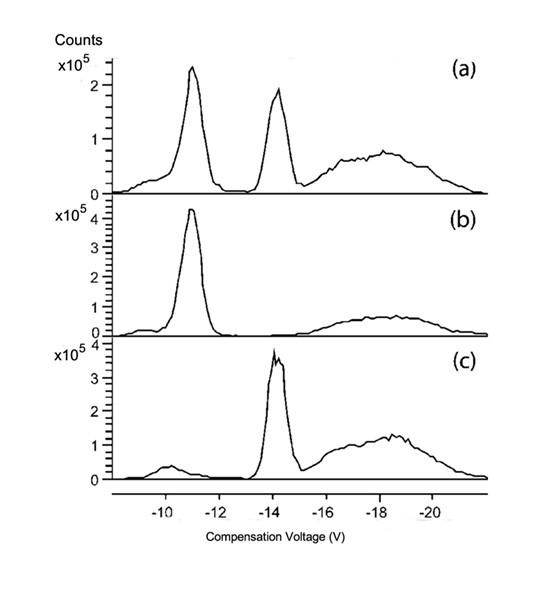
Figure 1. Separation of (+)/(-)-terbutaline as trimeric cluster ions [Cu(L-Trp)2(terb)-H]+. (a) Mixture of (+) and (-)-terbutaline; (b) (+)-terbutaline individually; and (c) (-)-terbutaline individually. The curves show extracted ion chromatograms of m/z 226 from MS/MS fragmentation of parent in m/z 695. Fragment mass corresponds to [terb + H]+. Capillary exit, 75.0 V; skimmer 1, 35.0 V. Carrier gas N2/He 56%:44%. CV scan rate 0.33 V/min.
Separation of positional isomers.
Another example are positional isomers, which differ in the location of bonding of some side-group.
We consider here poymeric compounds, including polypeptides, in which the order of the substituent groups may vary. Peptides which differ in the location of an amino acid have identical mass and cannot be separated by mass spectrometry (MS), but may be observed in MS/MS. Figure 2 illustrates the separation of two peptides that differ by location of a side-group on one amino acid within the sequence of eight amino acids. In this example the carrier gas was several mixtures of N2 and hydrogen Shvartsburg and Smith.
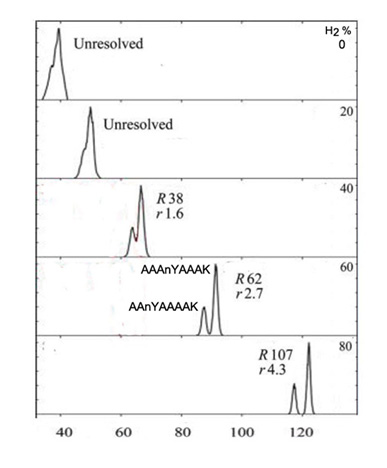
Figure 2. The tryptic peptide nYAAAAAAK (782 Da), where nY is nitrotyrosine, has seven tryptic sequence isomers. Shown is separation of the 1+ protonated isomers AAnYAAAAK and AAAnYAAAK (with nY shifted by single residue) using N2 with 0-80% H2 at filtering times of 0.2 s. When separated, AAnYAAAAK appears at lower CV.
........................ to be continued, work in progress